Guanidinoacetate methyltransferase (GAMT)
1. Guanidinoacetate methyltransferase (GAMT) catalyzes the creatine biosynthesis
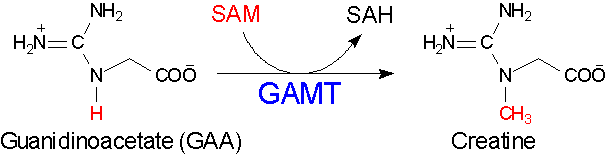
_________________________________________________________________________________________
2. Structure of Guanidinoacetate Methyltransferase (GAMT)

_________________________________________________________________________________________
3. Catalytic mechanism based on
the crystal structures and site-directed mutagenesis
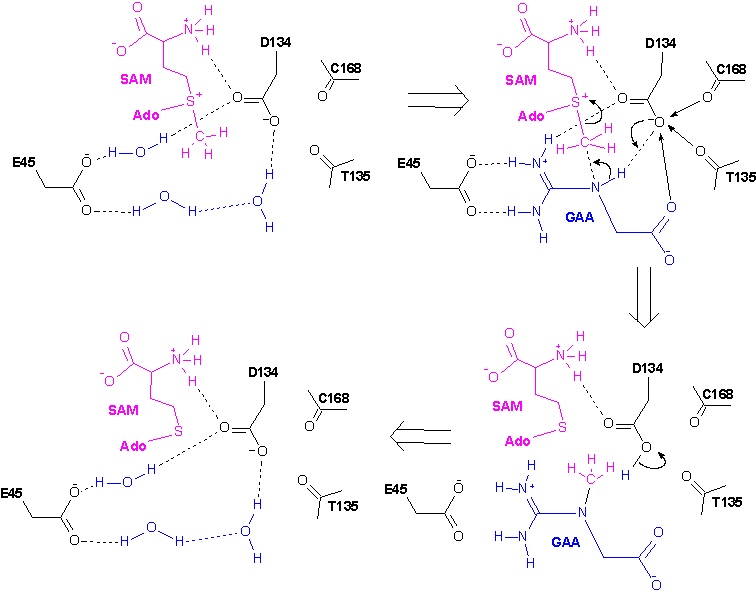
_________________________________________________________________________________________
4. N-terminus truncated GAMT has weak protein arginine methyltransferase activity
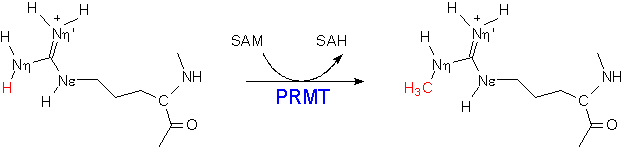
_________________________________________________________________________________________
5. A dimeric structure of the N-terminus truncated GAMT showing a protein arginine methyltransferase complex
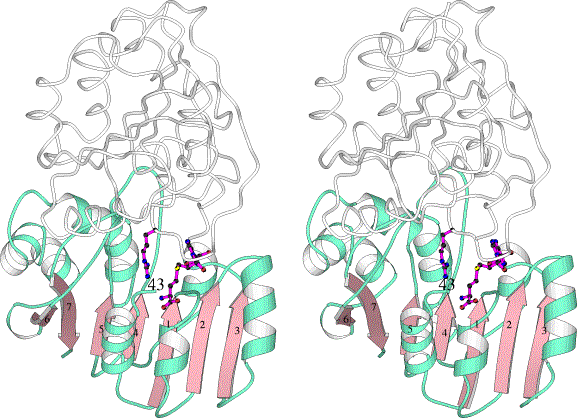
_________________________________________________________________________________________
Publications:
● Komoto, J.,
Huang, Y., Hu, Y., Takata, Y., Konishi, K., Ogawa, H., Gomi, T., Fujioka, M.
& Takusagawa, F. (1999). Crystallization and Preliminary X-ray
Diffraction Studies of Guanidinoacetate Methyltransferase from Rat Liver. Acta
Crystallogr. D55, 1928-1929.
● Komoto, J.,
Huang, Y., Takata, Y., Yamada, T., Konishi, K., Ogawa, H., Gomi, T., Fujioka,
M. & Takusagawa, F. (2002). Crystal Structure of Guanidinoacetate
Methyltransferase from Rat Liver: N-Terminus Truncated Enzyme Represents A
Ternary Structure of Protein Arginine Methyltransferase. J. Mol. Biol. 320, 223-235.
● Komoto, J.,
Takata, Y., Yamada, T., Ogawa, H., Gomi, T., Fujioka, M. & Takusagawa,
F. (2003). Monoclinic guanidinoacetate methyltransferase and gadolinium
ion-binding characteristics. Acta Crystallogr D Biol Crystallogr. 59, 1589-1596.
● Komoto, J., Yamada, T., Takata, Y., Konishi, K., Gomi, T., Ogawa,
H., Fujioka, M. & Takusagawa, F. (2004). Catalytic mechanism of
guanidinoacetate methyltransferase: Crystal structures of guanidinoacetate
methyltransferase ternary complexes. Biochemistry 43,14385-1494.